

_ electron groups on N Tro: Chemistry: A Molecular Approach, 2/e.VSEPR groups: Regions of Valence Electrons Each lone pair of electrons constitutes one electron group on a central atom Each bonding region constitutes one electron group on a central atom regardless of whether it is single, double, or triple O N VSEPR Theory Electron groups around the central atom repel each other (same “-” charge) Electron groups are spaced away to minimize the repulsion –valence shell electron pair repulsion theory (VSEPR theory) VSEPR theory can predict the shapes and bond angles in the molecule Tro: Chemistry: A Molecular Approach, 2/e.Lewis theory predicts there are regions of electrons in an atom Electron groups (= VSEPR group): Bonding pairs and Lone pairs Bonding pairs: Shared pairs of valence electrons between bonding nuclei Nonbonding pairs (= Lone Pairs): Unshared valence electrons on a single nuclei Tro: Chemistry: A Molecular Approach, 2/e
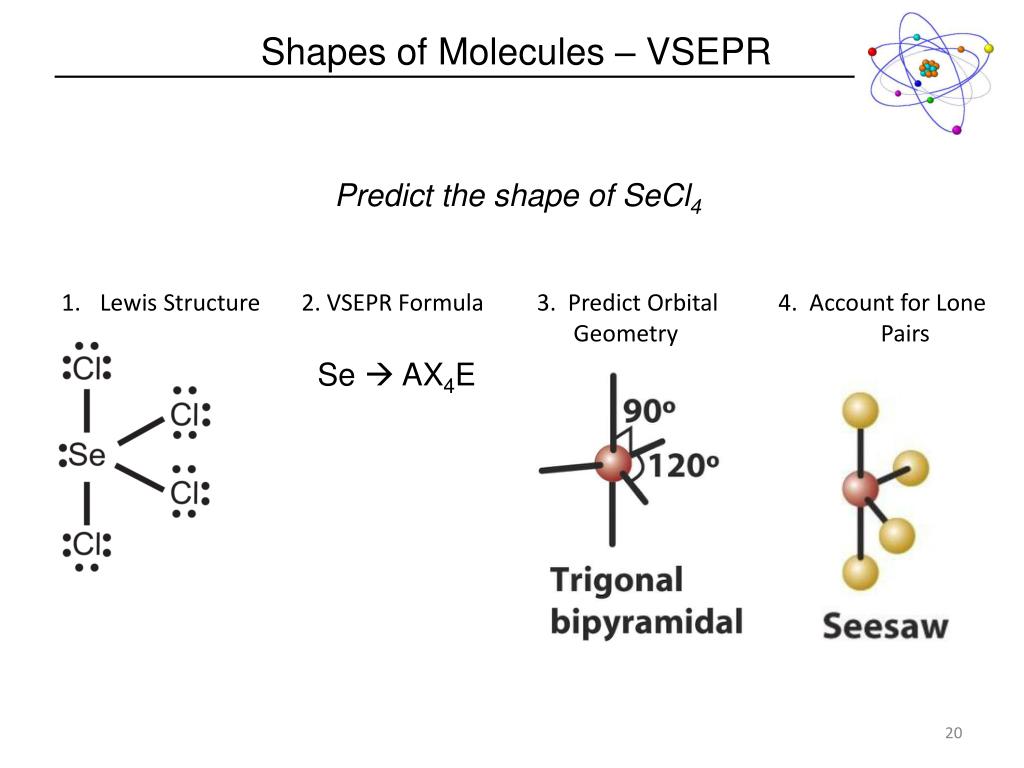
Molecular Geometry: Shape of a molecule Two important factors in Molecular Geometry: Bond Angle Bond Length Tro: Chemistry: A Molecular Approach, 2/e Properties of molecular substances depend on the structure of the molecule The structure includes many factors, such as: the skeletal arrangement of the atoms the kind of bonding between the atoms ionic, polar covalent, or covalent the shape of the molecule Bonding theory should allow you to predict the shapes of molecules Tro: Chemistry: A Molecular Approach, 2/eĥ Molecular Geometry Molecules: 3-dimensional objects Sugar molecules (“Key”) fit into the active site of taste cell receptors (“Lock”): When the sugar molecule enters the active site, parts of the taste cell receptor split apart ion channels in the cell membrane to open resulting in nerve signal transmission Artificial sweeteners also fit into the same receptor, sometimes binding even stronger than sugar (making them “sweeter” than sugar) Tro: Chemistry: A Molecular Approach, 2/e Hence, a trigonal planar molecule (BF3) is nonpolar because the bond polarities cancel each other, but a trigonal pyramidal molecule (NH3) is polar.Presentation on theme: "VSEPR theory Molecular Polarity"- Presentation transcript:Ģ Taste The taste of a food: Interaction between food molecules and taste cells Factors: Shape of the molecule and charge distribution within the molecule Food or “spicy” molecule fit snugly into the active site of specialized proteins on the surface of taste cells When this happens, changes in the protein structure cause a nerve signal Tro: Chemistry: A Molecular Approach, 2/e

Hence, a trigonal planar molecule (BF3) is nonpolar because the bond polarities cancel each other, but a trigonal pyramidal molecule (NH3) is polar. A molecule that is symmetrical and the atoms attached to the central atom have almost same electronegativity, then the molecule will be nonpolar. It all depends on symmetry and electronegativity concept. Shape:Ī tetrahedral molecule can be polar or nonpolar. The remaining four atoms connected to the central atom gives the molecule a square planar shape….Square Planar.

The three factors governing polarity are shape, electronegativity and dipole moment. Tetrahedral is polar depending on geometry and can also be nonpolar. The shape of the molecule will determine the direction of each of the individual bond dipoles, and thus, will always play a role in determining the polarity of the molecule as a whole. How does molecular shape relate to polarity?ġ Answer.


 0 kommentar(er)
0 kommentar(er)
